Surface Tension
Surface Tension: Overview
This topic covers concepts, such as, Surface Tension,Cohesive Force and Surface Tension,Application of Surface Tension etc.
Important Questions on Surface Tension
Water rises to a height of in a capillary tube and mercury falls to a depth of in the same capillary tube. If the density of mercury is and its angle of contact is and density of water is and its angle of contact is , then the ratio of surface tensions of the two liquids is
The radii of two soap bubbles are and . In isothermal conditions, two meet together in vacuum. Then the radius of the resultant bubble is given by
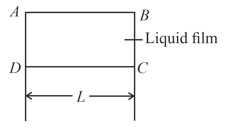
A film of water is formed between two straight parallel wires of length each separated by . if their separation is increased by while still maintaining their parallelism, how much work will have to be done (surface tension of water )
Radius of a soap bubble is increased from to work done in this process in terms of surface tension is
Radius of a soap bubble is increased from to work done in this process in terms of surface tension is
A liquid film is formed over a frame as shown in figure. Wire can slide without friction. The mass to be hung from to keep it in equilibrium is
There is horizontal film of soap solution. On it a thread is placed in the form of a loop. The film is pierced inside the loop and the thread becomes a circular loop of radius . If the surface tension of the loop be , then what will be the tension in the thread
A long wire is placed horizontally on the surface of water and is gently pulled up with a force of to keep the wire in equilibrium. The surface tension in of water is
A wooden stick long is floating on the surface of water. The surface tension of water . By putting soap solution on one side of the stick, the surface tension is reduced to . The net force on the stick will be
Mosquito eggs can float on water because of its _____.
Adding detergent in water _____ the surface tension of water and enhances the cleasing action of water and detergent both.
The surface tension of antiseptic ointments is low which make them to spread over the wound.This statement is true or false.
What are the two application of surface tension?
With the rise in temperature, cohesive force will ____.
On the surface of the liquid in equilibrium, molecules of the liquid possess
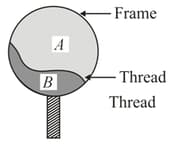
A thread is tied slightly loose to a wire frame as shown in the figure, and the frame is dipped into a soap solution and taken out. The frame is completely covered with the film. When the portion is punctured with a pin, the thread :
In a cylinder piston arrangement, air is under a pressure and a soap bubble of radius lies inside the cylinder. The soap bubble has a surface tension . Now, the radius of soap bubble is reduced to half by increasing the pressure of air up to . Choose the correct option(s).
How do sailors protect their ship during a heavy storm?
Surface tension causes the spherical nature of a water drop.
A glass tube has inner diameter of , outer diameter of . It is kept vertical and partially dipped in water. Calculate the downward pull due to surface tension. (Surface tension of water)
